
1. Expected use
Used as an adjuvant therapy to reduce the frequency.
2. Product composition
This product consists of two main parts:
3. Technical specifications
Power supply:
4. Display:
2.0-inch TFT screen
Resolution: 320 × 240
Brightness: 250-280 cd/m ²
5. Product safety classification:
BF type application part
6. Host output current pulse parameters
Output mode:
This product has three output modes:
Mode A: 20Hz ± 1Hz
B mode: 25Hz ± 1Hz
M Mode: Mindfulness Channel (Meditation Mode)
Output stimulus parameters:
1. Stimulus intensity:
Adjustable 0-5mA, with an error of ± 10%
2. Pulse frequency:
Mode A: 20Hz ± 1Hz
B mode: 25Hz ± 1Hz
3. Pulse width:
0.25 milliseconds ± 10%
0.5 milliseconds ± 10%
7. Electrode performance
1. Cell toxicity test: No toxic reaction detected (level 0).
2. Allergy test: No allergic reactions.
3. Skin irritation test: minimal irritation.
8. Wire performance
Total length of electrode wire: 1150mm (tolerance ± 5%)
Electrode plug length: 31.5mm (tolerance ± 5%)
Connecting wire cross-sectional area: not less than 0.05mm ²
9. Equipment classification
This product belongs to mobile medical equipment and is operated through a dedicated mobile terminal.
Embedded software can customize stimulation parameters to provide corresponding pulse outputs.

1. Expected use
Used as an adjuvant therapy to reduce the frequency.
2. Product composition
This product consists of two main parts:
3. Technical specifications
Power supply:
4. Display:
2.0-inch TFT screen
Resolution: 320 × 240
Brightness: 250-280 cd/m ²
5. Product safety classification:
BF type application part
6. Host output current pulse parameters
Output mode:
This product has three output modes:
Mode A: 20Hz ± 1Hz
B mode: 25Hz ± 1Hz
M Mode: Mindfulness Channel (Meditation Mode)
Output stimulus parameters:
1. Stimulus intensity:
Adjustable 0-5mA, with an error of ± 10%
2. Pulse frequency:
Mode A: 20Hz ± 1Hz
B mode: 25Hz ± 1Hz
3. Pulse width:
0.25 milliseconds ± 10%
0.5 milliseconds ± 10%
7. Electrode performance
1. Cell toxicity test: No toxic reaction detected (level 0).
2. Allergy test: No allergic reactions.
3. Skin irritation test: minimal irritation.
8. Wire performance
Total length of electrode wire: 1150mm (tolerance ± 5%)
Electrode plug length: 31.5mm (tolerance ± 5%)
Connecting wire cross-sectional area: not less than 0.05mm ²
9. Equipment classification
This product belongs to mobile medical equipment and is operated through a dedicated mobile terminal.
Embedded software can customize stimulation parameters to provide corresponding pulse outputs.
1. Expected use
Used as an adjuvant therapy to reduce the frequency.
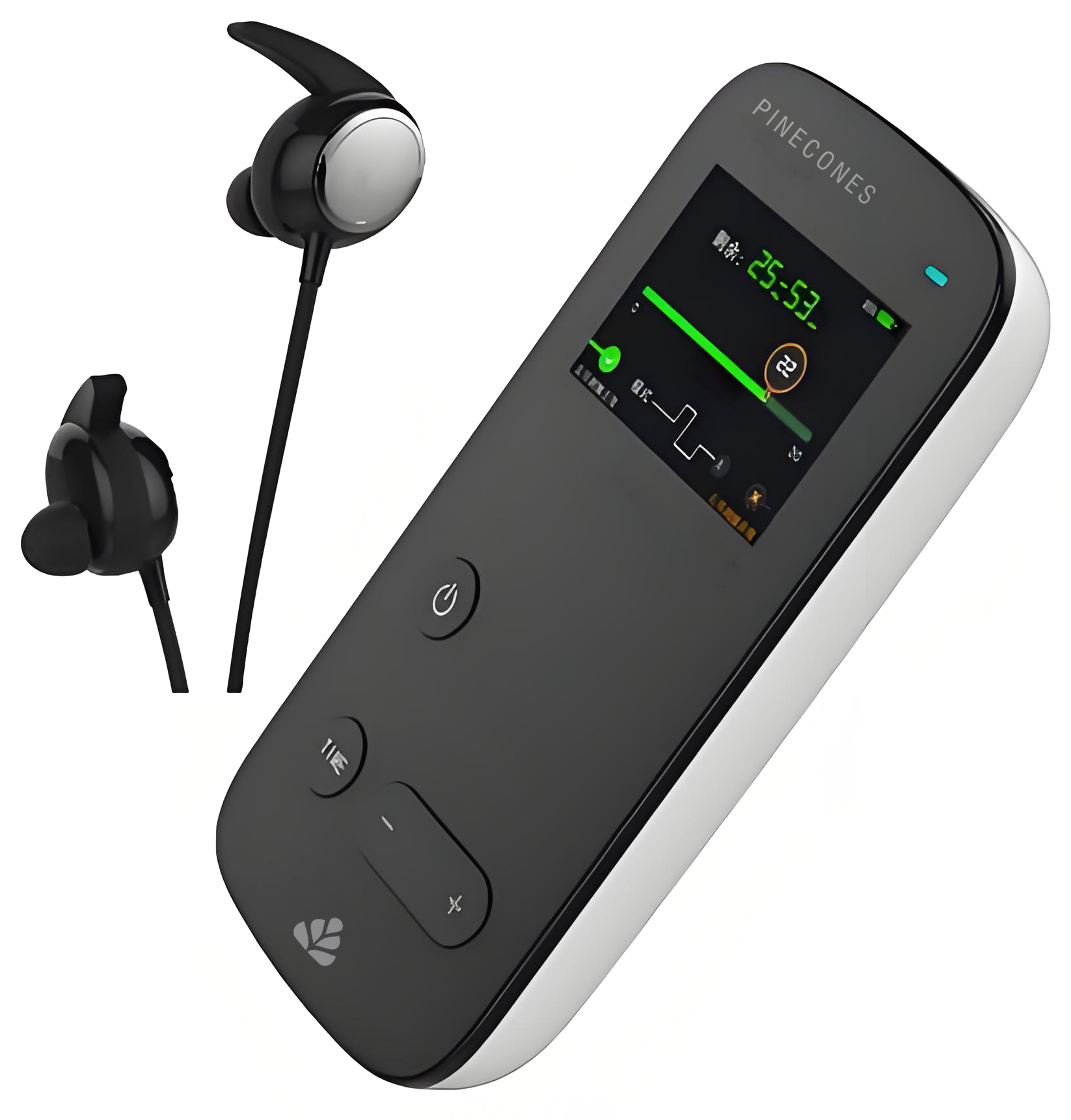
2. Product composition
This product consists of two main parts:
3. Technical specifications
Power supply:
4. Display:
2.0-inch TFT screen
Resolution: 320 × 240
Brightness: 250-280 cd/m ²
5. Product safety classification:
BF type application part
6. Host output current pulse parameters
Output mode:
This product has three output modes:
Mode A: 20Hz ± 1Hz
B mode: 25Hz ± 1Hz
M Mode: Mindfulness Channel (Meditation Mode)
Output stimulus parameters:
1. Stimulus intensity:
Adjustable 0-5mA, with an error of ± 10%
2. Pulse frequency:
Mode A: 20Hz ± 1Hz
B mode: 25Hz ± 1Hz
3. Pulse width:
0.25 milliseconds ± 10%
0.5 milliseconds ± 10%
7. Electrode performance
1. Cell toxicity test: No toxic reaction detected (level 0).
2. Allergy test: No allergic reactions.
3. Skin irritation test: minimal irritation.
8. Wire performance
Total length of electrode wire: 1150mm (tolerance ± 5%)
Electrode plug length: 31.5mm (tolerance ± 5%)
Connecting wire cross-sectional area: not less than 0.05mm ²
9. Equipment classification
This product belongs to mobile medical equipment and is operated through a dedicated mobile terminal.
Embedded software can customize stimulation parameters to provide corresponding pulse outputs.